THE WHAT? The Delhi High Court recently delivered a verdict in a three-year legal tussle between two of India’s most popular moisturiser brands, NIVEA and Ponds. Beiersdorf AG, the manufacturer of NIVEA, filed a case against Hindustan Unilever Limited (HUL), which produces Ponds, alleging unfair market practices and trademark infringement.
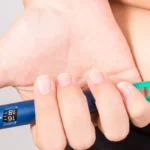
THE DETAILS In 2021, Beiersdorf AG accused Ponds salespersons of engaging in deceptive practices by comparing NIVEA and Ponds creams in a way that misrepresented NIVEA’s product. The sales tactic involved applying NIVEA’s cream on one hand and Ponds on the other, using a magnifying glass to show customers that NIVEA’s cream left more residue. Beiersdorf sought a permanent injunction against these practices, claiming trademark infringement and market disparagement.
HUL argued that they were using a generic blue tub without NIVEA’s branding, asserting that NIVEA does not hold a monopoly on the colour blue. They also defended their product comparison, stating that Ponds’ cream was less sticky. NIVEA countered, stating that the comparison was unfair as it pitted a heavy cream with 25% fatty matter against a light gel with 10% fatty matter. They suggested that a more appropriate comparison would be with NIVEA Men fresh gel, which has similar fat content to Ponds’ super light gel.
Justice Anish Dayal ruled in favour of NIVEA, stating that HUL’s practices were misleading and disparaging, causing irreversible prejudice to NIVEA. The court also dismissed HUL’s defence on the colour blue, ruling that the similarity in packaging was likely intended to confuse consumers and associate Ponds’ products with NIVEA’s.
THE WHY? This legal battle underscores the importance of fair competition and trademark protection in the beauty industry. The court’s decision not only protects NIVEA’s brand identity but also sets a precedent for addressing misleading marketing practices. This ruling reinforces the need for transparency and honesty in product comparisons, ensuring that consumers make informed choices based on accurate information.
Clinical aesthetics products refer to a category of products used in the field of medical aesthetics or cosmetic dermatology. These products are typically designed and formulated to be used under the supervision of healthcare professionals, such as dermatologists, plastic surgeons, or trained aestheticians. They are distinct from over-the-counter cosmetics in that they often contain active ingredients or formulations that require expertise in their application or administration.
Examples of clinical aesthetics products include:
-
Dermal Fillers: Injectable substances used to add volume, smooth wrinkles, and enhance facial contours. Examples include hyaluronic acid fillers like Juvederm and Restylane.
-
Botulinum Toxin (Botox): Injectables that temporarily paralyze facial muscles to reduce the appearance of wrinkles caused by repetitive movements, such as frown lines and crow's feet.
-
Chemical Peels: Solutions applied to the skin to exfoliate and improve its texture. They can treat acne, pigmentation issues, and signs of aging.
-
Laser and Light Therapies: Devices that emit focused light or laser energy to treat various skin conditions, including acne, scars, and signs of aging.
-
Prescription Skincare Products: Formulations containing active ingredients like retinoids (vitamin A derivatives), hydroquinone, or prescription-strength antioxidants to address specific skin concerns under medical supervision.