Did you know that the global rate of obesity has more than doubled since 1990, and adolescent obesity has quadrupled? These alarming statistics, highlighted by the World Health Organization, underscore the growing prevalence of weight-related concerns on a global scale. Various weight loss solutions have emerged as the number of individuals dealing with weight concerns continues to rise.
Fortunately, Wegovy, a prescription weight loss medication, has obtained approval from the US Food and Drug Administration (FDA). This approval underscores its safety and efficacy in addressing the weight-related concerns of both obese and overweight adults and adolescents aged 12 and up.
This article will explore the Wegovy FDA approval, its safety profile, reported side effects, and clinical data highlighting its safety and effectiveness.
Key Takeaways
- The US Food and Drug Administration (FDA) approved Wegovy injections to help adults with obesity or overweight and obese adolescents aged 12 and older with their weight-related concerns.
- Wegovy stands out for its superior weight reduction and cardioprotective effects compared to other antidiabetic medications.
- The US Food and Drug Administration (FDA) evaluates new medications or products to ensure their safety and effectiveness.
- It’s worth noting that the prescribing information of Wegovy contains a warning to inform healthcare professionals and patients about the risk of thyroid C-cell tumors.
- When common side effects persist, or adverse events occur, patients must seek immediate medical attention to properly manage symptoms and avoid further risks.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to buy Wegovy wholesale, our dedicated sales agents can give you proper guidance. We offer a worry-free experience in searching for the best and most popular products on the market. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Understanding Wegovy and its FDA Approval
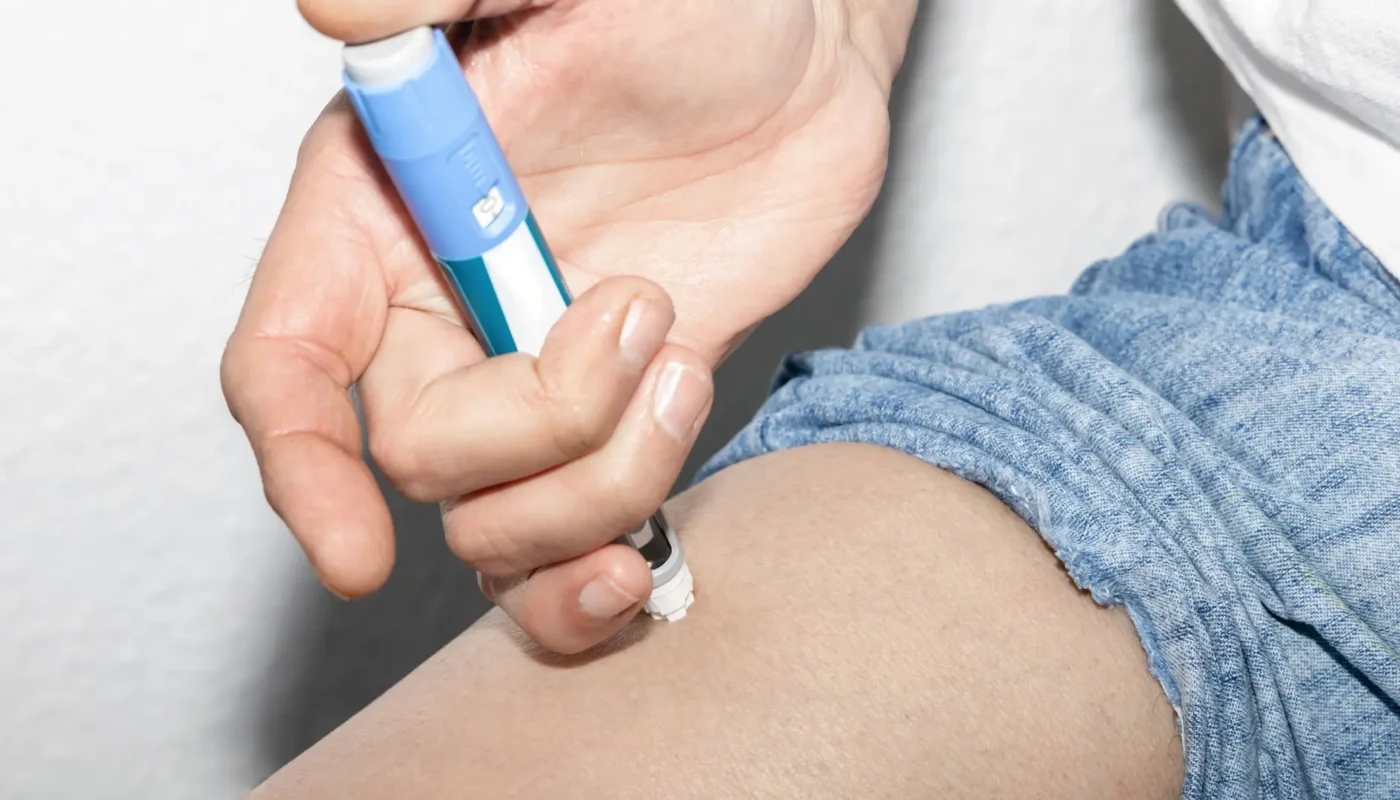
Wegovy is an injectable prescription medication used for weight loss management. It contains semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist. This mimics the natural hormone in the body that regulates appetite, leading to less calorie intake and weight loss. This injectable requires a once-weekly subcutaneous injection into the thigh, arm, or abdomen sites.
The US Food and Drug Administration (FDA) approved Wegovy injections to help adults with obesity or overweight and obese adolescents aged 12 and older with their weight-related concerns. Additionally, the prescription injectable received approval for reducing the risk of major cardiovascular events in adults with heart diseases who are either obese or overweight.
The treatment requires a combination of Wegovy injections, a reduced-calorie diet, and increased physical activity for optimal weight loss outcomes. Earning the US FDA’s approval involves a thorough process, as the agency ensures the safety and efficacy of products and medications before individuals use them.
Despite the Wegovy FDA approval, individuals must understand that side effects and Wegovy pen malfunctions may still occur. Furthermore, the US FDA approval signifies a medication’s benefits overshadow its known and potential risks for the intended population and indications.
Clinical Trials and Studies
According to a clinical trial for weight reduction and long-term maintenance studies in adults with obesity or overweight, after 68 weeks, Wegovy treatment led to a significant decrease in body weight compared to the placebo group.
Meanwhile, changes in body weight at week 68 for obesity or overweight with comorbidity after a 20-week run-in resulted in greater proportions of patients treated with Wegovy achieving 5%, 10%, and 15% weight loss than those treated with placebo.
In a 68-week clinical trial targeting weight reduction and long-term maintenance in pediatric patients aged 12 years and older with obesity, more patients treated with Wegovy achieved a greater than 5% reduction in baseline BMI compared to those treated with placebo.
Wegovy stands out for its superior weight reduction and cardioprotective effects compared to other antidiabetic medications. Additionally, semaglutide offers the advantage of long-term weight management. With Wegovy’s recent approval, patients anticipate enhanced weight loss outcomes and improved quality of life for patients.
According to Novo Nordisk’s safety profile of Wegovy, 6.8% of adult patients treated with Wegovy and 3.2% of patients treated with placebo permanently discontinued treatment due to adverse reactions. Medical professionals should equip and discuss this information and safety profile with patients, allowing them to understand the treatment, its potential risks, and benefits to make informed healthcare decisions.
Regulatory Process and Approval Criteria

The US Food and Drug Administration (FDA) evaluates new medications or products to ensure their safety and effectiveness. For weight management medications like Wegovy, the meticulous approval process may involve the following:
- Discovery or Concept: Pharmaceutical companies explore and identify potential drug candidates.
- Preclinical Research: Laboratory and animal tests are conducted to understand how the drug works and assess safety.
- Clinical Research: At this stage, independent experts conduct human trials to evaluate safety, efficacy, and health benefits.
- FDA Review: The FDA’s Center for Drug Evaluation and Research (CDER) reviews data submitted by the drug maker. If benefits outweigh known risks, approval is granted.
- FDA Post-Market Safety Monitoring: After approval, the FDA continues to monitor the drug’s safety and effectiveness.
Including this regulatory process, the US FDA also considers other factors for approving a new drug or product: the drug’s intended use and current treatment field, benefit-risk assessment based on well-designed clinical trials, and evaluating uncertainties or incomplete data.
Wegovy underwent a series of 68-week trials, demonstrating significant weight loss and reducing cardiovascular risks. Over 17,600 participants received either Wegovy or placebo. Wegovy significantly reduced the risk of major adverse cardiovascular events (cardiovascular death, heart attack, and stroke), which occurred in 6.5% of participants who received Wegovy compared to 8% of participants who received a placebo.
However, it’s worth noting that the prescribing information of Wegovy contains a warning to inform healthcare professionals and patients about the risk of thyroid C-cell tumors. The contraindications of Wegovy injection include the following:
- Personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN2).
- Known hypersensitivity to semaglutide or any of the excipients in Wegovy
Post-Marketing Surveillance and Reported Side Effects


Wegovy underwent rigorous clinical trials before the US FDA approved its medication. However, real-world and post-marketing surveillance of the Wegovy pens reveals additional insights about the injectables. According to the Prescribing Information, post-marketing experience includes adverse events, such as gastrointestinal disorders and hypersensitivity.
- Heart Health Benefits: Recently, the Wegovy injections received US FDA approval. Even without substantial weight loss, Wegovy improves heart health, potentially tying the potency of an anti-obesity drug and heart health medication.
- Severe Stomach Issues: Among GLP-1 receptor agonists, the risk of gastrointestinal adverse events has increased with these injectables. According to the study, these include pancreatitis, gastroparesis, and bowel obstruction but not biliary disease.
- Thyroid Tumors: Wegovy carries a potential risk for thyroid cancer, although the exact impact on humans remains uncertain.
The common and reported Wegovy side effects include nausea, diarrhea, vomiting, constipation, stomach (abdomen) pain, headache, tiredness (fatigue), upset stomach, dizziness, feeling bloated, belching, low blood sugar in people with type 2 diabetes, gas, stomach flu, heartburn, and runny nose, or sore throat.
Serious side effects can include possible thyroid tumors, pancreatitis, gallbladder issues, kidney problems, and allergic reactions. When common side effects persist, or adverse events occur, patients must seek immediate medical attention to properly manage symptoms and avoid further risks.
Conclusion
The Wegovy FDA approval marks a significant milestone in the battle against obesity. It offers a promising option for individuals struggling with weight-related concerns. The thorough evaluation process by the FDA underscores the medication’s safety and efficacy, reassuring healthcare professionals and patients.
With its proven ability to induce weight reduction and potential cardioprotective effects, Wegovy presents a valuable addition to the weight management tools. However, patients and healthcare providers must be mindful of possible side effects and Wegovy pen malfunctions while remaining vigilant in monitoring and managing any adverse reactions.
FAQs
1. What is Wegovy?
Wegovy is a prescription weight loss medication approved by the US Food and Drug Administration (FDA) to help adults with obesity or overweight and obese adolescents aged 12 and older with their weight-related concerns. It contains semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, and is administered via a once-weekly subcutaneous injection.
2. How does Wegovy stand out compared to other weight management medications?
Wegovy stands out for its superior weight reduction and cardioprotective effects compared to other antidiabetic medications. The medication’s approval by the US Food and Drug Administration (FDA) underscores its efficacy and safety in addressing weight-related concerns for adults with obesity or overweight and obese adolescents aged 12 and older.
3. What should patients and healthcare professionals know about the Wegovy FDA approval?
While the FDA approval of Wegovy signifies its benefits in addressing weight-related concerns, it’s essential to be mindful of potential side effects and pen malfunctions that may occur. Patients and healthcare providers should stay informed about the safety profile of Wegovy and be prepared to manage any adverse reactions effectively.
References
- World Health Organization. (2024, March 1). Obesity and overweight. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- FDA. (2024, March 8). FDA Approves First Treatment to Reduce Risk of Serious Heart Problems Specifically in Adults with Obesity or Overweight. FDA. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-reduce-risk-serious-heart-problems-specifically-adults-obesity-or
- Wegovy® Safety Profile | Wegovy® (semaglutide) injection 2.4 mg. (n.d.). NovoMEDLINK. Retrieved June 27, 2024, from https://www.novomedlink.com/obesity/products/treatments/wegovy/efficacy-safety/safety-profile.html
Clinical aesthetics products refer to a category of products used in the field of medical aesthetics or cosmetic dermatology. These products are typically designed and formulated to be used under the supervision of healthcare professionals, such as dermatologists, plastic surgeons, or trained aestheticians. They are distinct from over-the-counter cosmetics in that they often contain active ingredients or formulations that require expertise in their application or administration.
Examples of clinical aesthetics products include:
-
Dermal Fillers: Injectable substances used to add volume, smooth wrinkles, and enhance facial contours. Examples include hyaluronic acid fillers like Juvederm and Restylane.
-
Botulinum Toxin (Botox): Injectables that temporarily paralyze facial muscles to reduce the appearance of wrinkles caused by repetitive movements, such as frown lines and crow's feet.
-
Chemical Peels: Solutions applied to the skin to exfoliate and improve its texture. They can treat acne, pigmentation issues, and signs of aging.
-
Laser and Light Therapies: Devices that emit focused light or laser energy to treat various skin conditions, including acne, scars, and signs of aging.
-
Prescription Skincare Products: Formulations containing active ingredients like retinoids (vitamin A derivatives), hydroquinone, or prescription-strength antioxidants to address specific skin concerns under medical supervision.